
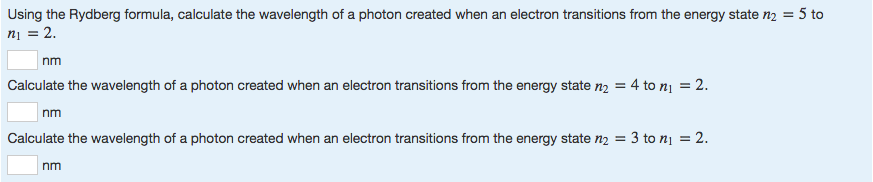
Determine the ν¯ for the transition wherein n1=6 to n2 = 3 in a hydrogen atom. The modern value of Rydberg constant is 109677.57 cm-1 and the most precise known physical constant. N1 and n2 are integers and n2 is always greater than n1. Is the Rydberg constant always greater than N2? λ= Wavelength of the emmited light (electromagnetic rediation) in the vacuum R = Rydberg Constant (1.097x 107 m-1) Z = Number of proton in the nucleus of the element nf = Principal quantum number of final state ni = Principal quantum number of the initial state. Johann Balmer, a Swiss mathematician, discovered (1885) that the wavelengths of the visible hydrogen lines can be expressed by a simple formula: the reciprocal wavelength (1/λ) is equal to a constant (R) times the difference between two terms, 1/4 (written as 1/22) and the reciprocal of the square of a variable integer … Which is the correct formula for the Rydberg equation? The transitions are named sequentially by Greek letter: n = 3 to n = 2 is called H-α, 4 to 2 is H-β, 5 to 2 is H-γ, and 6 to 2 is H-δ. The Balmer series is characterized by the electron transitioning from n ≥ 3 to n = 2, where n refers to the radial quantum number or principal quantum number of the electron. The Rydberg formula is a mathematical formula used to predict the wavelength of light resulting from an electron moving between energy levels of an atom. When an element’s gaseous state is heated, it will give off light. What is Rydberg formula give its application? According to Paschen series, n1 = 3 and n2 = 4, 5… λ = 1.282 x 10-4 cm = 1282 nm which is in near infrared region. The modern value of Rydberg constant is known as 109677.57 cm-1 and it is the most accurate physical constant.


Theory: When an electron in an atom receives some energy by any means, it moves to a bigger radius orbit whose energy level fits that electron�s energy. What does N stand for in Rydberg equation?Īnswer: In a model of a hydrogen atom where n means the energy level (n=1 is the ground state where the electron is closest to the nucleus n=2 is an excited state where the electron is farther from the nucleus n=3 is another excited state where the electron is even farther from the nucleus…), you can use the … How do you solve for the Rydberg constant?Ģ) to calculate Rydberg�s constant, R = 1.


 0 kommentar(er)
0 kommentar(er)
